When doctors are examining suspected cancer cases, they turn to biomarker tests to help make a diagnosis.
With a testing system and patient samples, physicians can investigate potential leads, narrowing down their list of culprits to provide precise, effective treatment for patients.

A team led by Florida State University chemists has developed a new test for detecting biological markers related to several types of cancer. Their research was recently published in Journal of the American Chemical Society.
“Better tools for detecting cancer mean more effective treatment for patients,” said study co-author Hedi Mattoussi, a professor in the FSU Department of Chemistry and Biochemistry. “Our goal in this research was to build a biosensor that would light up in the presence of cancer markers, offering another tool for the ongoing problem of detecting this disease.”
The sensing platform is made of a gold nanoparticle and molecules called peptides that are labeled with a dye. The components are connected by chemical bonds, and the gold nanoparticle keeps the dye from glowing in the presence of UV light. When a patient sample containing the enzyme MMP-14 — a biomarker for various types of cancers, but most commonly for breast cancer — is added, it breaks bonds in the peptides, separating a fragment with the dye from the gold. Without the gold to absorb the energy from the dye, the sample begins to glow.
“You start with a system that is dark, which we can think of as ‘off,’ like we would with a light switch,” Mattoussi said. “When you bring in the enzyme, the system turns ‘on’ and emits light. It is like a beacon.”
The light glowing from the sample depends on the concentration of the enzyme and interaction time. By measuring that light, researchers can generate data that inform them if a cancer marker is present in a sample and in what levels.
Various tests already exist for examining whether a patient has cancer. This research is a first step toward developing a method that can test for a wider variety of cancers.
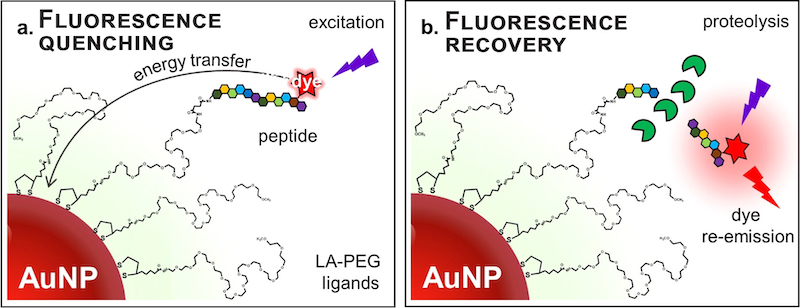
An illustration of how the biomarker testing process developed by the research team works. Peptides are connected to a gold nanoparticle (AuNP) and to dye. Energy from the dye is transferred to the nanoparticle, which prevents it from glowing under ultraviolet light — a phenomenon known as energy quenching. When an enzyme is added to the testing set-up, it severs the bond between the peptide and the gold nanparticle, allowing it to glow under UV light. The light glowing from the sample depends on the concentration of the enzyme and interaction time. By measuring that light, researchers can generate data that inform them if a cancer marker is present in a sample and in what levels. (Courtesy of Hedi Mattoussi)
Mattoussi and his team tested their system with the MMP-14 enzyme but they plan to expand this research with work that matches more peptide chains with other enzyme cancer markers. With further development, it could allow scientists to use a single assessment to test for a variety of cancers at once.
“The platform we have designed is applicable to any enzyme,” he said. “All that you need to do is vary the nature of the peptide that is attached to the nanoparticle. You change the peptide, you change the enzyme, and it works the same way.
Along with its applications for detecting disease, the research also sheds more light on how enzymes interact with peptides that are attached to nanoparticles and how the addition of enzymes affects the testing system.
“One aspect of our research was the application aspect,” Mattoussi said. “Cancer diagnosis is still a huge problem. Early detection has been a limiting factor. But the other aspect of this work was the ability to develop a platform that starts with a nanoparticle attached to a peptide labeled with a dye and understand the way they interact. How efficient are those interactions? What happens when you vary the size of nanoparticles or the number of peptides? So, there is a practical aspect and a fundamental aspect to this research.”
Co-authors on this paper were FSU researchers: Zhicheng Jin, a former FSU doctoral student who is now a postdoctoral associate at Harvard University, Narjes Dridi, Goutam Palui, who is now a researcher at the U.S. Food and Drug Administration, and Professor Qing-Xiang “Amy” Sang; Valle Palomo and Philip E. Dawson, of The Scripps Research Institute; and Jesse V. Jokerst of University of California San Diego.
This work was funded by the National Science Foundation, the Air Force Office of Scientific Research and Kasei-Asahi Corporation.
